Asher Bio is applying our cis-targeting platform to optimize the therapeutic potential of cytokines and other immunomodulators.
We are focused on progressing our lead cis-targeted immunotherapy program, etakafusp alfa (formerly known as AB248), through clinical development. Etakafusp alfa is a novel CD8+ T cell selective interleukin-2 (IL-2), specifically engineered to activate and expand tumor-killing CD8+ T-cells while avoiding natural killer (NK) cells, which can act as a pharmacological sink and contribute to toxicity, and also avoiding regulatory T (Treg) cells, which are immunosuppressive.
Our broader cis-targeting pipeline includes AB821, a CD8-targeted interleukin 21 (IL-21), designed to selectively activate CD8+ T cells via a pathway distinct from and complementary to IL-2. IL-21 enables T cells to kill tumor cells more effectively by enhancing their cytotoxic function.
Beyond etakafusp alfa and AB821, we have rapidly developed and are advancing a pipeline of immunotherapies to address patient populations with unmet medical needs. While our primary focus is on oncology, the modular nature of our cis-targeting platform allows us to leverage other immunomodulators and immune cell types to expand into additional therapeutic areas including infectious diseases.
Given the potential applicability of our platform to a variety of targets and indications, we are exploring opportunities to enter into strategic collaborations to access capabilities outside our core areas of focus and accelerate delivering therapies to patients.
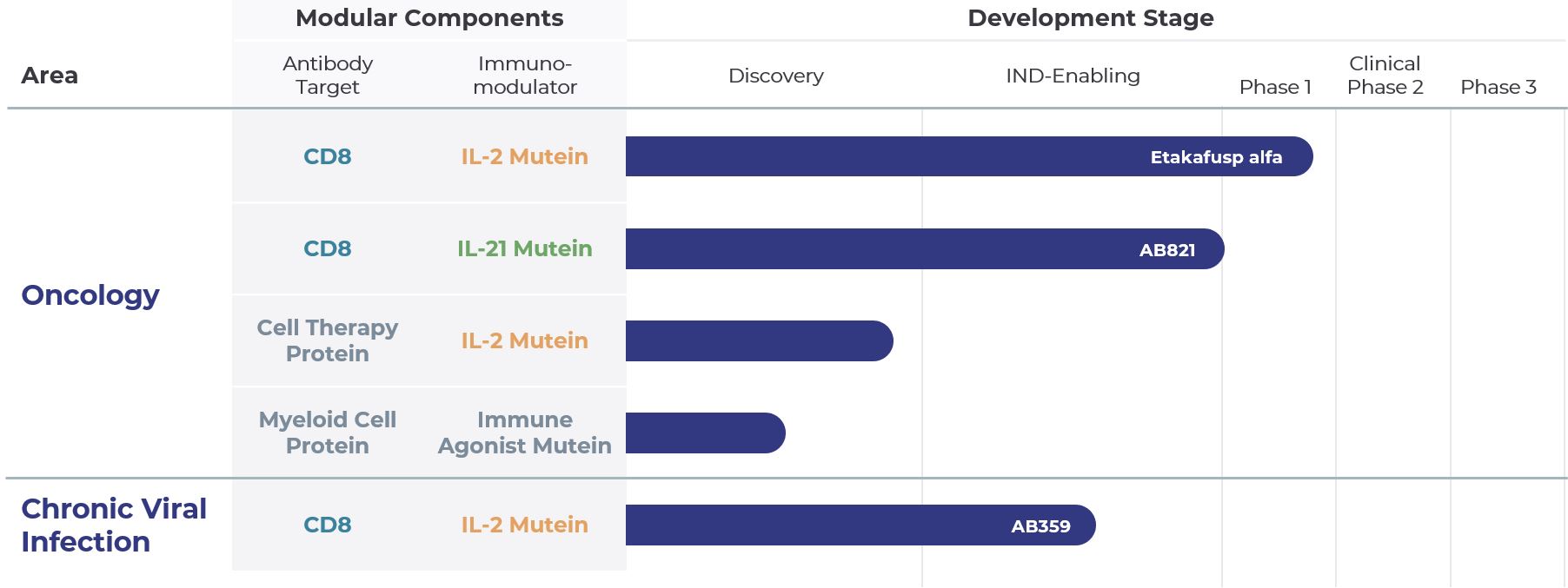
Etakafusp alfa is currently being studied in an open-label Phase 1a/1b clinical trial, which consists of a dose escalation and expansion stage, evaluating etakafusp alfa as a monotherapy and in combination with KEYTRUDA® (pembrolizumab) in patients with recurrent locally advanced or metastatic solid tumors, including melanoma, renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), and squamous cell carcinoma of the head and neck (SCCHN).
Please refer to www.clinicaltrials.gov (NCT05653882) for additional details related to this Phase 1a/1b clinical trial.
LEARN MORE ABOUT etakafusp alfa, OUR CD8-TARGETED IL-2 THERAPY
Our lead cis-targeted immunotherapy, etakafusp alfa, selectively activates the IL-2 receptor on CD8+ T cells stimulating proliferation. Initial pharmacokinetic and pharmacodynamic data from the ongoing Phase 1a/1b trial have demonstrated proof of mechanism and activity with a highly differentiated clinical profile.