Providing a selective cytokine signal to adoptively transferred T cell therapies is expected to enhance engraftment, persistence, and functionality of infused cells ultimately leading to improved therapeutic outcomes.
Chimeric antigen receptor T cell (CAR-T) therapies have transformed treatment for a subset of patients with highly refractory hematological malignancies. However, there remains an unmet need to expand treatment to patients unable to tolerate pre-conditioning and to patients with solid tumors which often express an immunosuppressive microenvironment.
In the clinic, successful expansion and persistence of CAR-T cells has correlated with therapeutic outcomes, including durable complete responses and survival. Administration of cytokine support, such as IL-2, enhances CAR-T engraftment, persistence, and functionality in preclinical models. However, the clinical potential of utilizing IL-2 with CAR-T therapies is limited using current molecules due to the severe toxicity of high-dose IL-2 and the inadequate selectivity of existing engineered IL-2 variants, which expand multiple endogenous cell types in addition to transferred CAR-T cells.
We believe that a highly selective cis-targeted cytokine fusion molecule that specifically activates only CAR-T cells by delivering either IL-2 or IL-21, while exhibiting minimal activity on bystander cells, may allow for superior efficacy and durability. This approach may also decrease the number of CAR-T cells required at infusion, reduce the need for preconditioning, and enable temporal control over cell activation.
The modular nature of our cis-targeting platform enables the potential to: a.) deliver different, supportive cytokine signals to cell therapies, and b.) target a diverse set of cell therapies including those based on TCR-T, TILs, NK cells, and Tregs. In preclinical models, our cell therapy cis-targeted IL-2 and IL-21 molecules both enhanced anti-tumor activity and did so via distinct mechanisms.
Cis-targeted cytokines for cell therapies provides a means to selectively boost the transferred cells.
The problem: some patients have poor CAR-T engraftment, expansion, and functionality, and this leads to poor patient outcome.
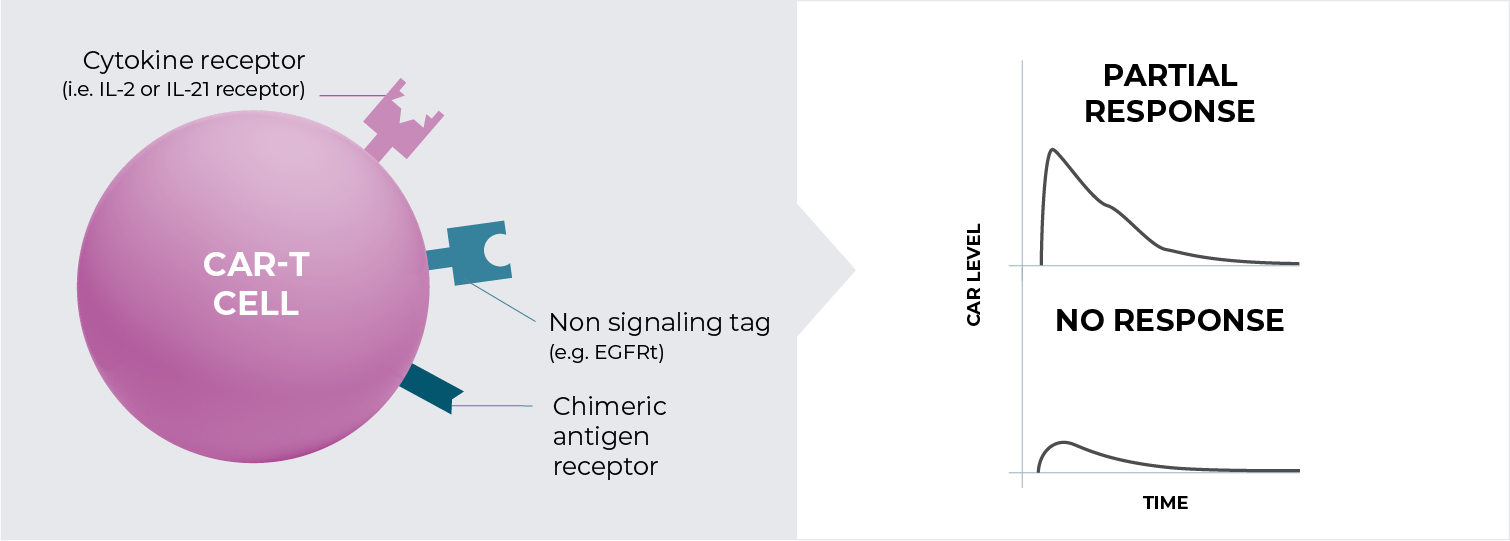
Adding cytokine support to CAR-T cells following infusion is expected to enhance engraftment, expansion, and functionality.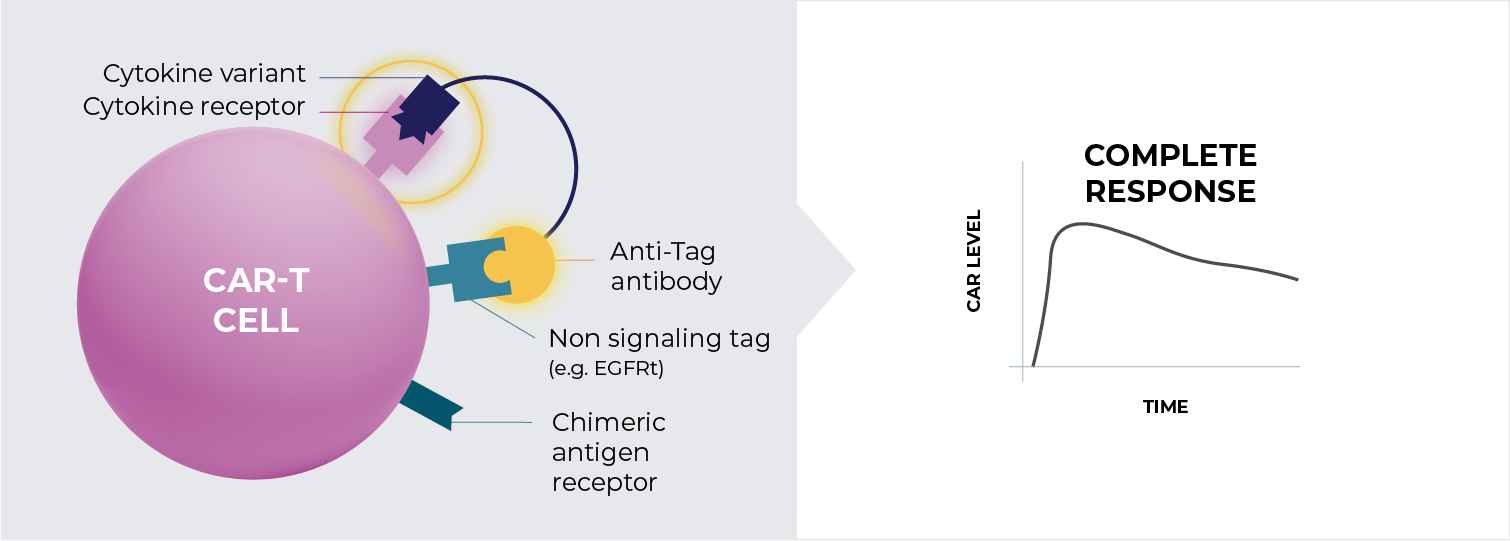
Our cis-targeted cytokine fusions are comprised of a targeting antibody directed against a tag expressed on the CAR-T surface, that is co-expressed with the CAR, and a cytokine mutein with attenuated binding to its cognate cytokine receptor.