IL-2 is a powerful T cell activator and an enticing immunotherapy that has yet to be optimized in the clinic. Asher Bio is pioneering a solution to maximize IL-2’s potential, employing cis-targeting technology to potently and selectively activate efficacy-driving CD8+ T cells while avoiding off-target toxicities.
Our lead cis-targeted immunotherapy, AB248, is a fusion protein that selectively activates the IL-2 pathway in CD8+ T cells, which are key drivers of anti-tumor efficacy. Maximizing activity of IL-2 on CD8+ T cells, while limiting its activity on immunosuppressive regulatory T cells (Tregs) and NK cells, which can contribute to toxicity, has the potential to improve on-target pharmacology, antitumor immunity, and enhanced tolerability.
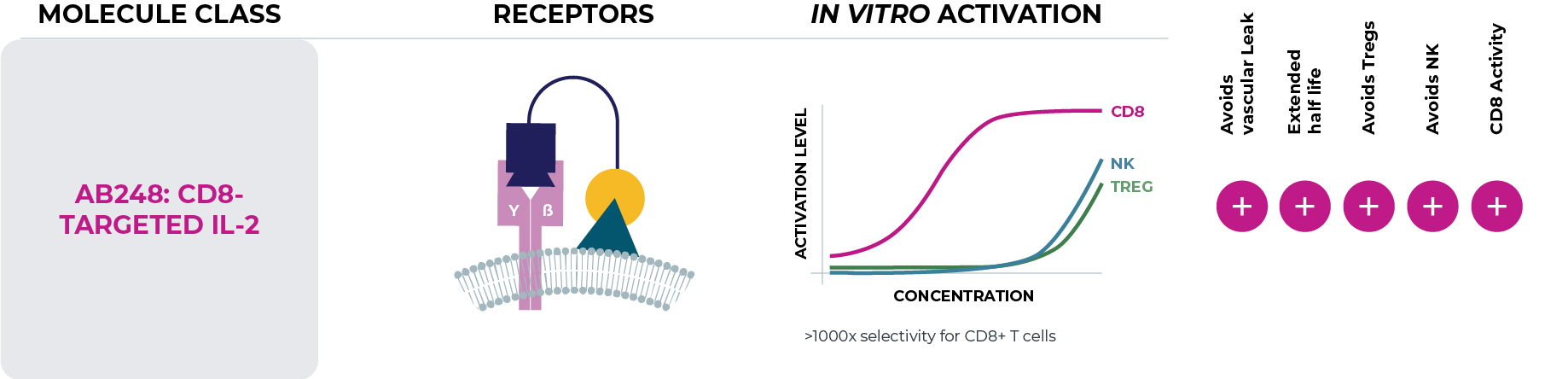
Initial pharmacokinetic and pharmacodynamic data from the ongoing Phase 1a/1b clinical trial support AB248’s proof of mechanism and activity with a highly differentiated clinical profile. Early data shows unprecedented levels of selectivity and CD8+ T cell activation without substantial changes to Treg and NK cell numbers, as well as initial evidence of anti-tumor activity with a well-tolerated safety profile. Asher Bio is currently investigating the use of AB248 in multiple solid tumor indications as monotherapy and in combination with PD(L)-1 checkpoint inhibitors.
In addition, AB248’s robust effect on CD8+ T cells may have therapeutic benefit in combination with cell therapies, vaccines and other immune-oncology modalities.
About AB248 Phase 1 Trial
AB248 is currently being studied in an open-label Phase 1a/b clinical trial consisting of a dose escalation and expansion phase to investigate the safety, pharmacokinetics (PK), pharmacodynamics (PD), and anti-tumor activity of AB248 alone and in combination with KEYTRUDA® (pembrolizumab) in subjects with locally advanced/metastatic solid tumors who failed prior therapies.
Upon determining a recommended dosing regimen, the study will open indication specific expansion cohorts to further evaluate monotherapy AB248 and the combination of AB248 and KEYTRUDA in patients with melanoma, RCC, and NSCLC after failure to prior therapies, as well as first-line SCCHN.
Please refer to www.clinicaltrials.gov (NCT05653882) for additional details related to this Phase 1a/1b clinical trial.