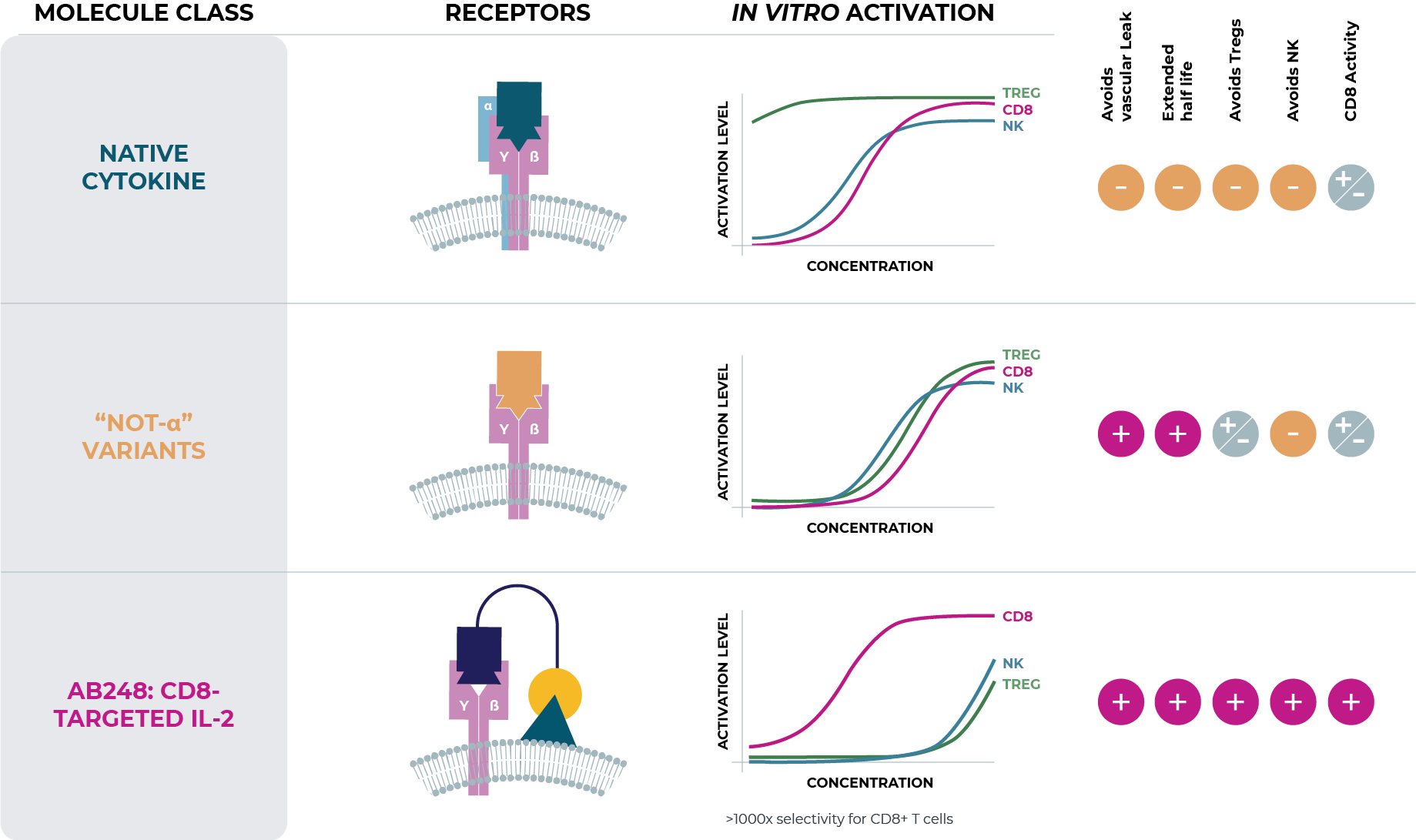
IL-2 is an enticing immunotherapy because it is a powerful T cell activator. However, as a therapeutic, the lack of selectivity has been a barrier, and its clinical use has been severely limited due to toxicity.
Our lead cis-targeted immunotherapy, AB248, is a fusion protein that selectively activates the IL-2 pathway in CD8+ T cells, which are key drivers of anti-tumor efficacy. Maximizing activity of IL-2 on CD8+ T cells, while limiting its activity on immunosuppressive regulatory T cells (Tregs) and NK cells, is expected to result in improved on-target pharmacology, antitumor immunity, and enhanced tolerability. AB248 has demonstrated a highly differentiated profile and compelling activity in multiple preclinical tumor models, showing an unprecedented level of selectivity and expansion of CD8+ T cells, leading to enhanced efficacy and reduced toxicities.Asher Bio plans to investigate the use of AB248 in multiple solid tumor indications as monotherapy and in combination with PD(L)-1 checkpoint inhibitors. In addition, AB248’s robust effect on CD8+ T cells may have therapeutic benefit in combination with cell therapies, vaccines and other immune-oncology modalities.
A Differentiated Approach
As recent data has supported a role for IL2Rα in many of IL-2’s liabilities, multiple biopharmaceutical companies initiated development programs recognizing the need for a more selective IL-2, with the hopes to fully realize the potent efficacy of IL-2 while solving its liabilities. Many of these next generation IL-2 programs are categorized as “not alpha” IL-2 variants.
In contrast to our approach, “not alpha” IL-2 variants’ attempts to obtain selectivity are inherently limited, as they rely on differential expression levels of the natural cytokine receptor across different cell types rather than achieving selectivity by design. In clinical studies, these molecules continue to activate multiple cell types, invoke dose-limiting toxicities, and demonstrate monotherapy efficacy lower than that historically achieved by wild-type IL-2 (aldesleukin). Therefore, the therapeutic opportunity to improve upon wild-type IL-2 remains.
AB248 is currently being studied in an open-label Phase 1a/1b clinical trial, which consists of a dose escalation and expansion stage, evaluating AB248 as a monotherapy and in combination with KEYTRUDA® (pembrolizumab) in patients with recurrent locally advanced or metastatic solid tumors, including melanoma, renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC) and squamous cell carcinoma of the head and neck (SCCHN).
Please refer to www.clincialtrials.gov (NCT05653882) for additional details related to this Phase 1a/1b clinical trial.